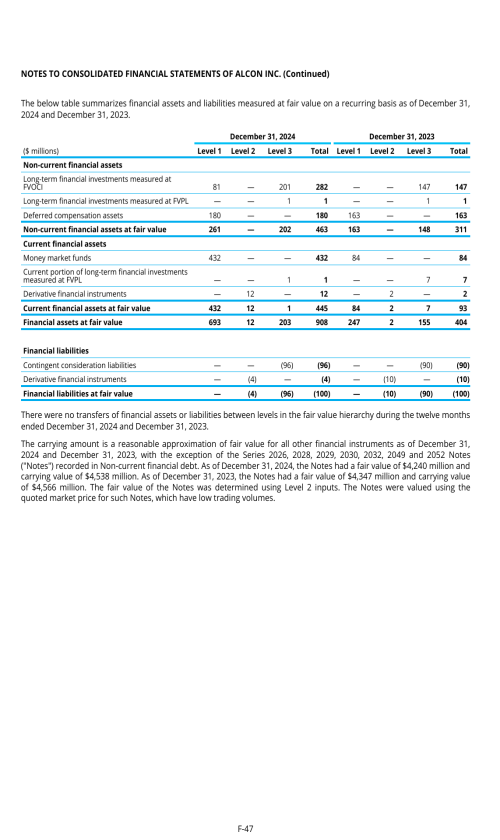
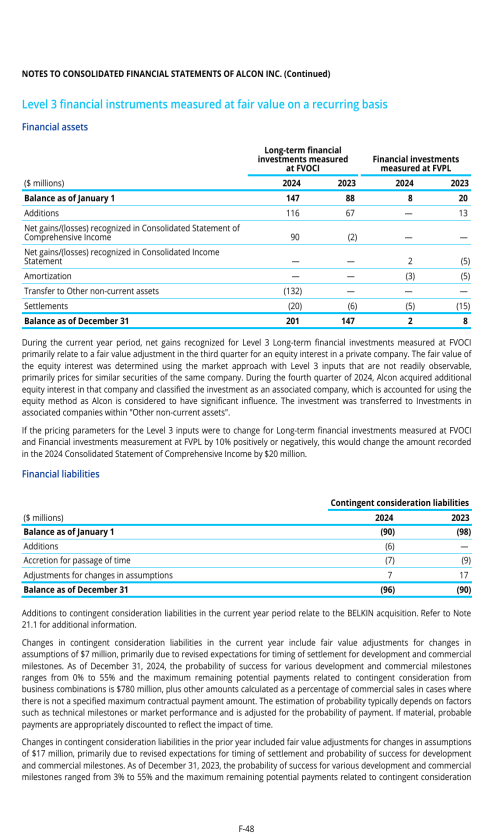
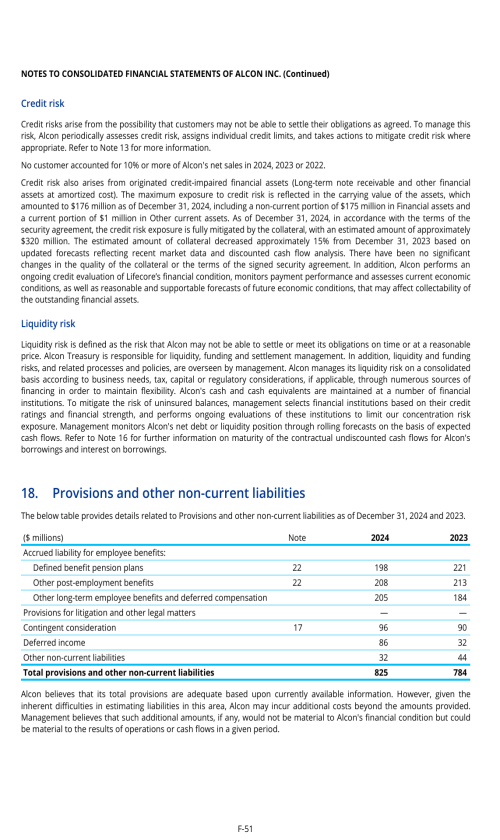
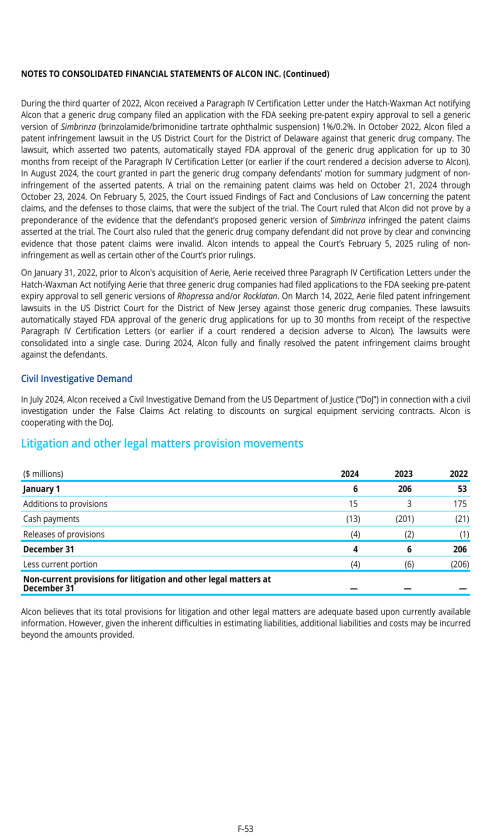
Index
Swiss Annual Report_Compiled for BoD Bring-down_2.24.2024
ALC 20F 2024 at Q4 (SWISS ANNUAL REPORT - BoD BRING-DOWN)
INTRODUCTION AND USE OF CERTAIN TERMS
MARKET INFORMATION
SPECIAL NOTE ABOUT FORWARD-LOOKING STATEMENTS
PART I
ITEM 1. IDENTITY OF DIRECTORS, SENIOR MANAGEMENT AND ADVISERS
1.A. DIRECTORS AND SENIOR MANAGEMENT
1.B. ADVISERS
1.C. AUDITORS
ITEM 2. OFFER STATISTICS AND EXPECTED TIMETABLE
ITEM 3. KEY INFORMATION
3.A. RESERVED
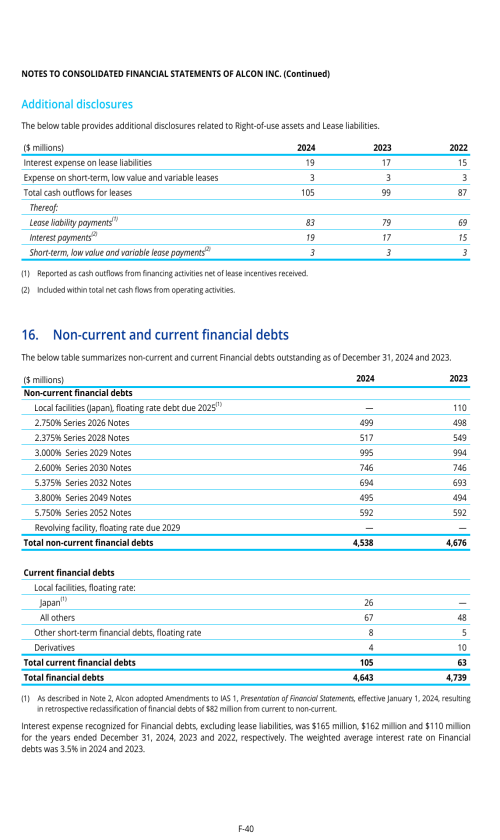
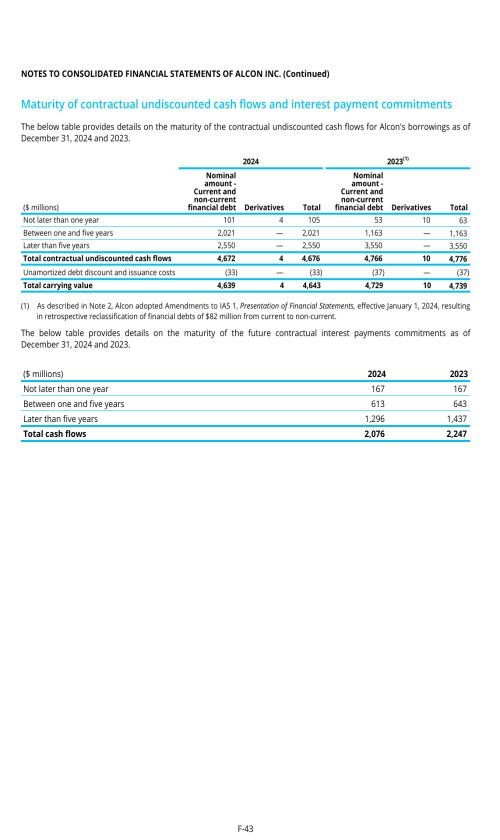
3.B. CAPITALIZATION AND INDEBTEDNESS
3.C. REASONS FOR THE OFFER AND USE OF PROCEEDS
3.D. RISK FACTORS
ITEM 4. INFORMATION ON THE COMPANY
4.A. HISTORY AND DEVELOPMENT OF THE COMPANY
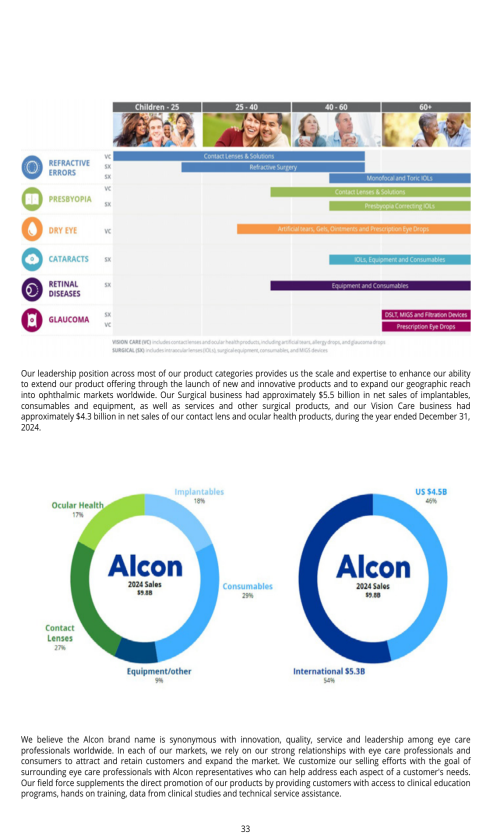
4.B. BUSINESS OVERVIEW
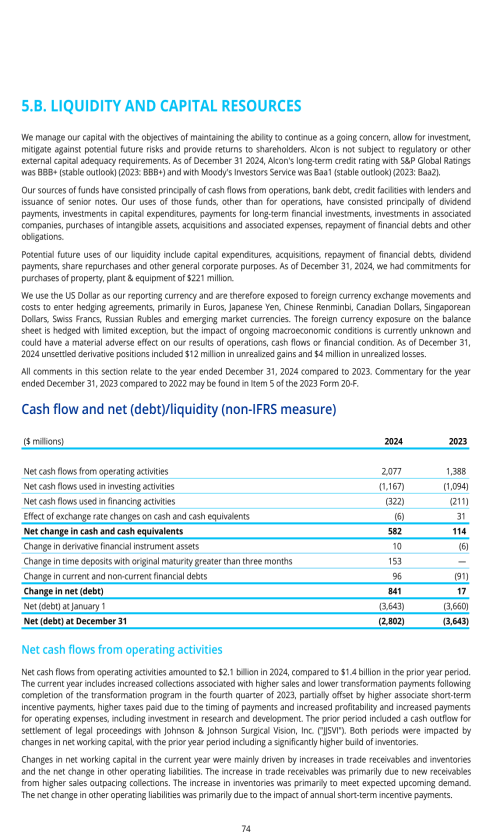
ITEM 5. OPERATING AND FINANCIAL REVIEW AND PROSPECTS
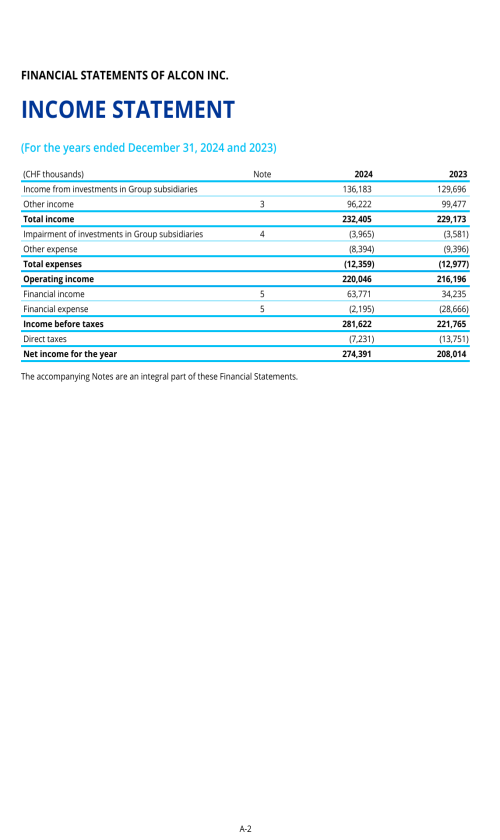
5.A. OPERATING RESULTS
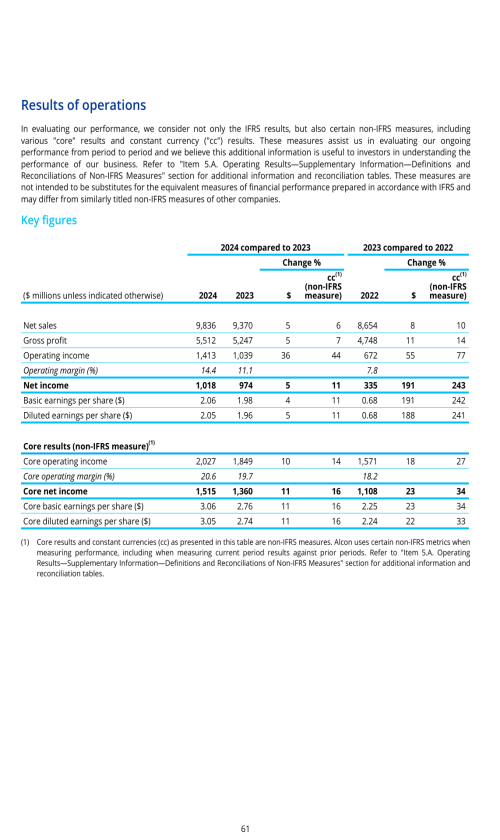
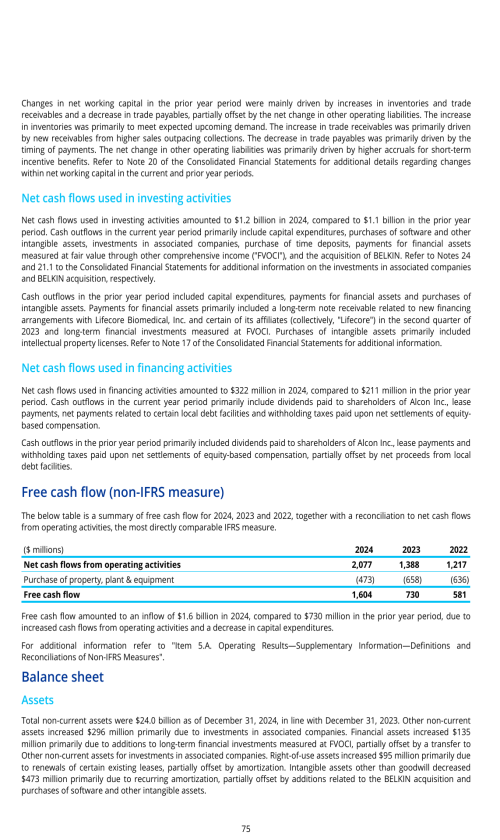
Overview
Basis of preparation
Items you should consider
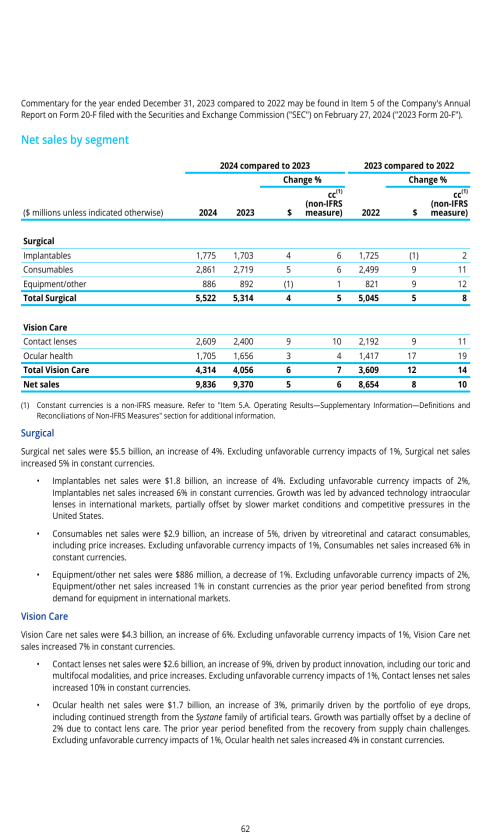
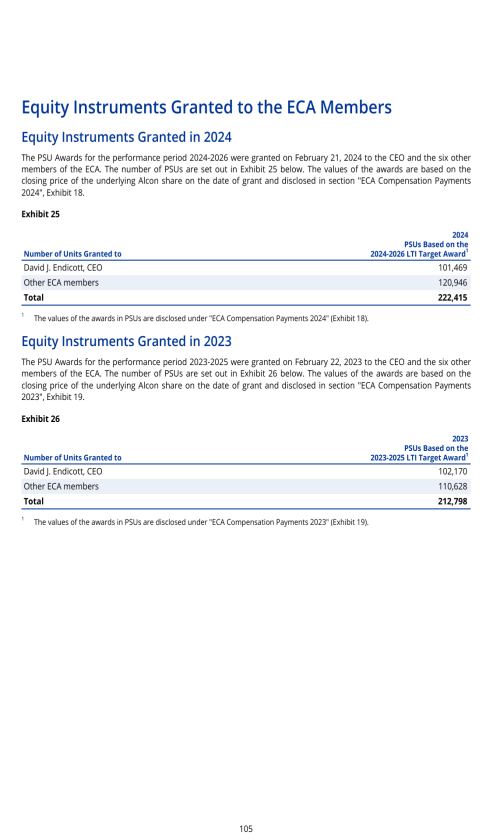
Segment description
Opportunity and risk summary
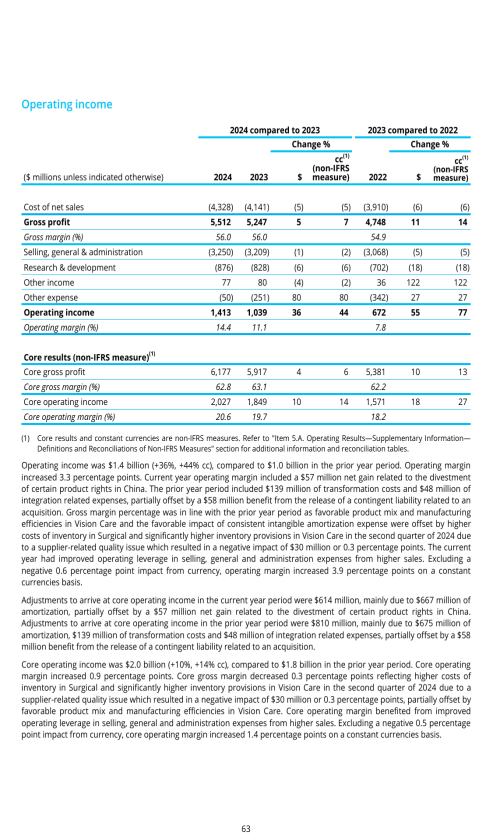
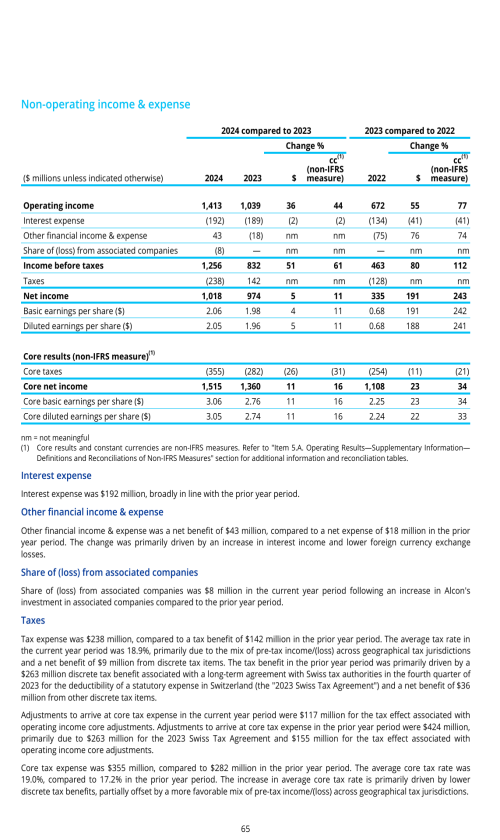
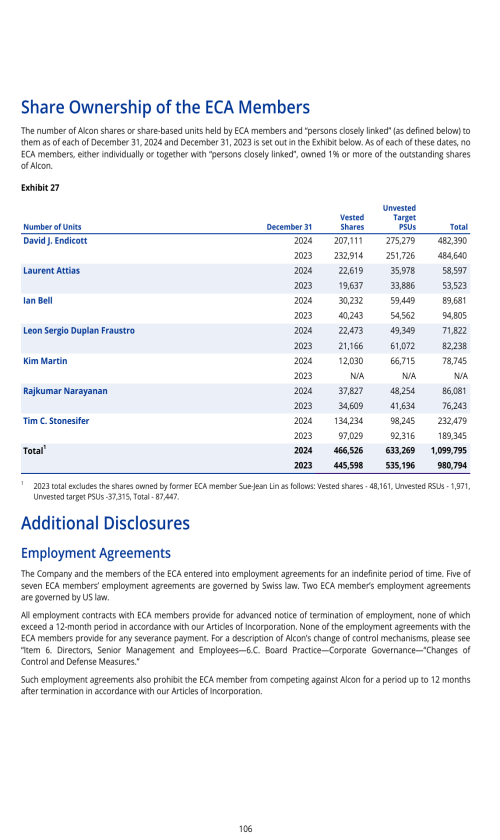
Components of results of operations
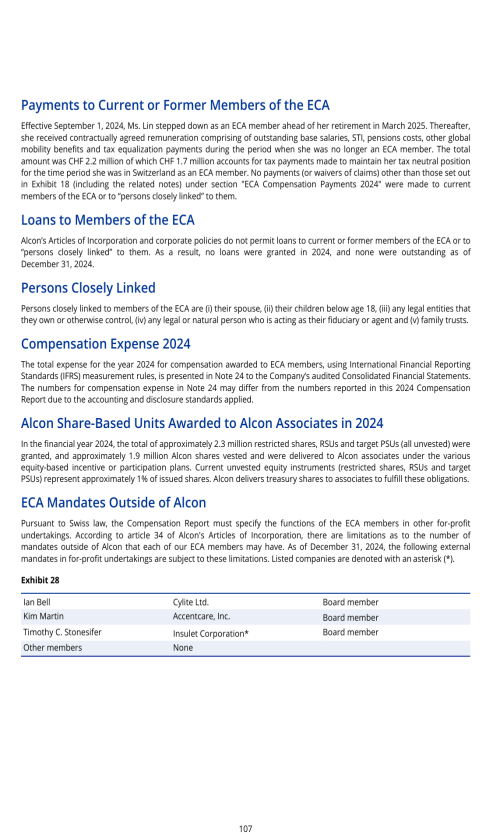
Critical accounting policies and estimates
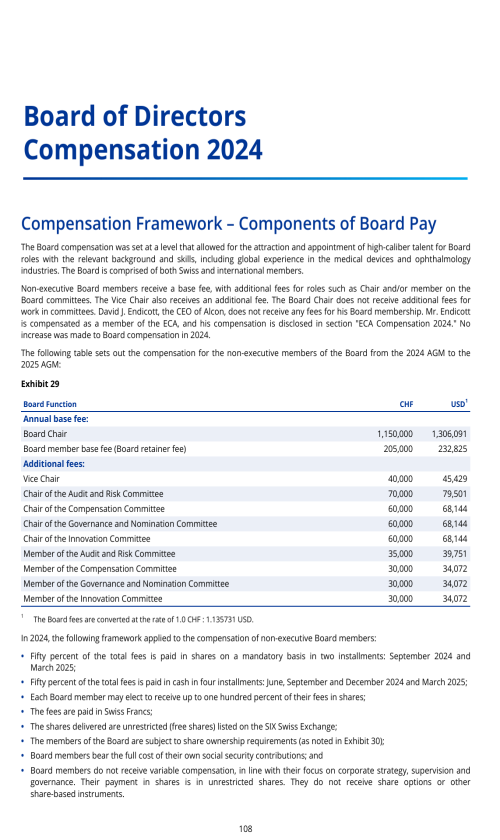
Factors affecting comparability of period to period results of operations
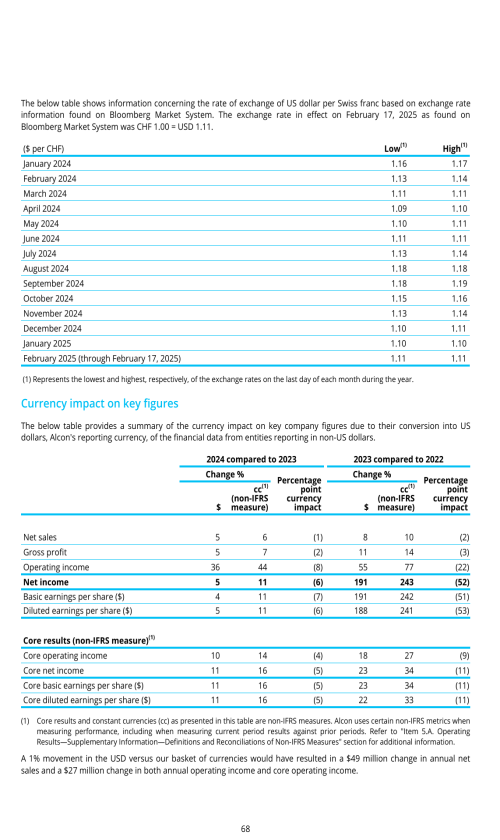
Effects of currency fluctuations
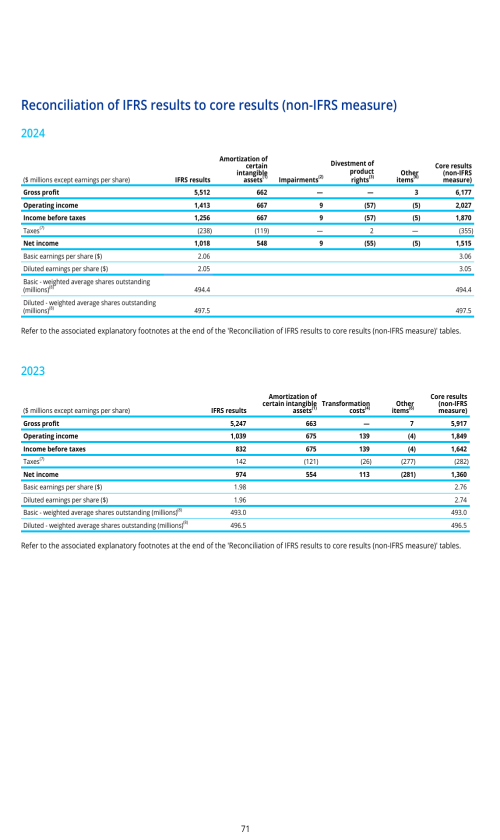
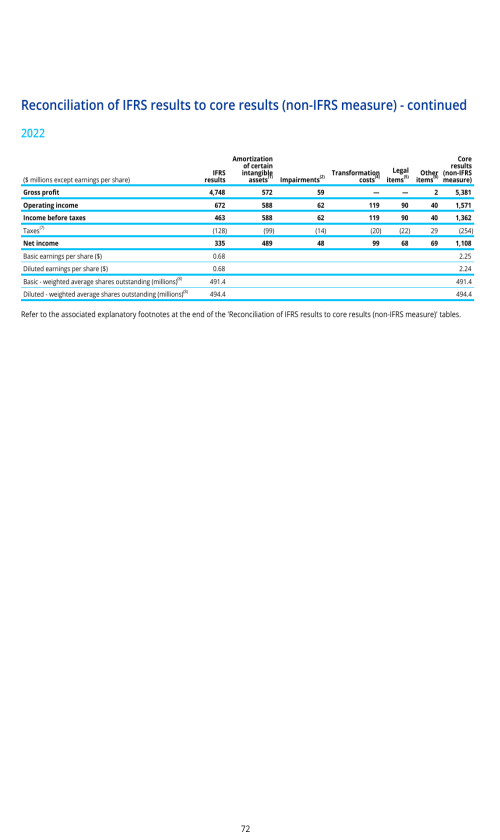
SUPPLEMENTARY INFORMATION - DEFINITIONS AND RECONCILIATIONS OF NON-IFRS MEASURES
Non-IFRS measures as defined by the Company
ITEM 6. DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES
6.A. DIRECTORS AND SENIOR MANAGEMENT
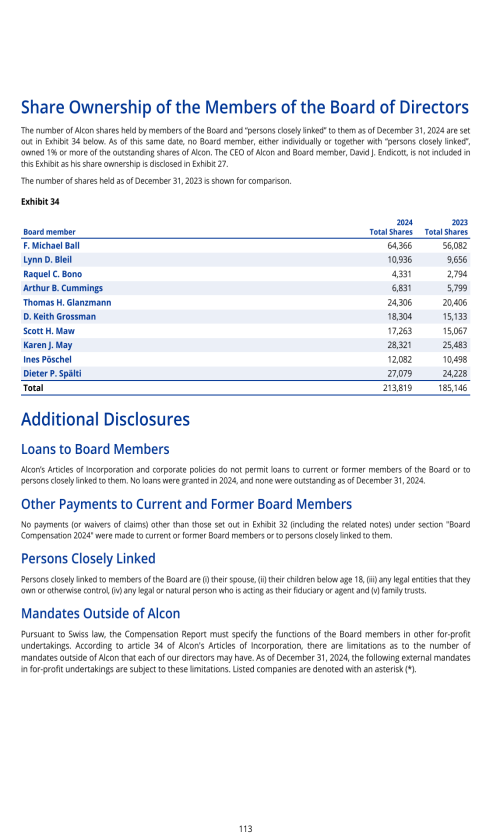
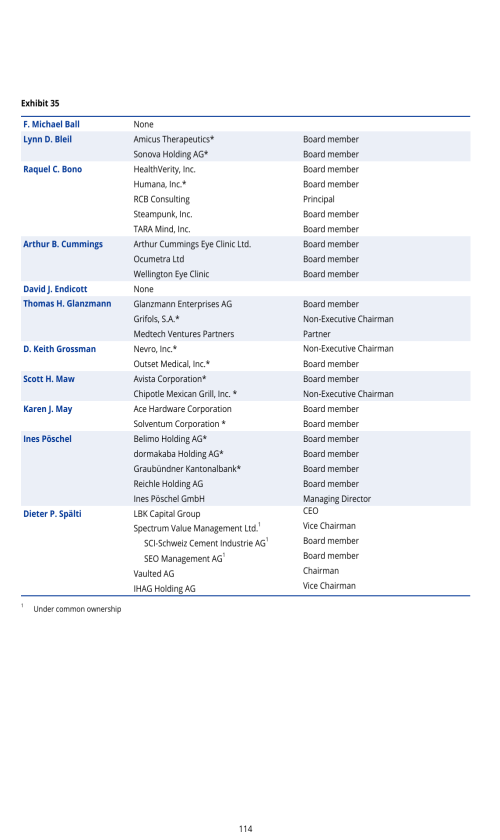
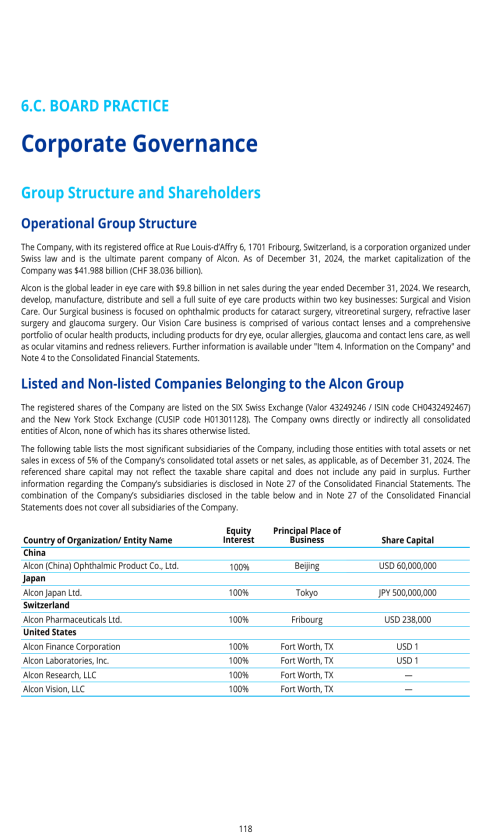
6.C. BOARD PRACTICES
Information and Control System of the Board vis-à-vis the Management
Information to the Board of Directors
Alcon Management Information System
Internal Audit
Internal Control System
Risk Management
Compliance Function
Changes of Control and Defense Measures
Auditors
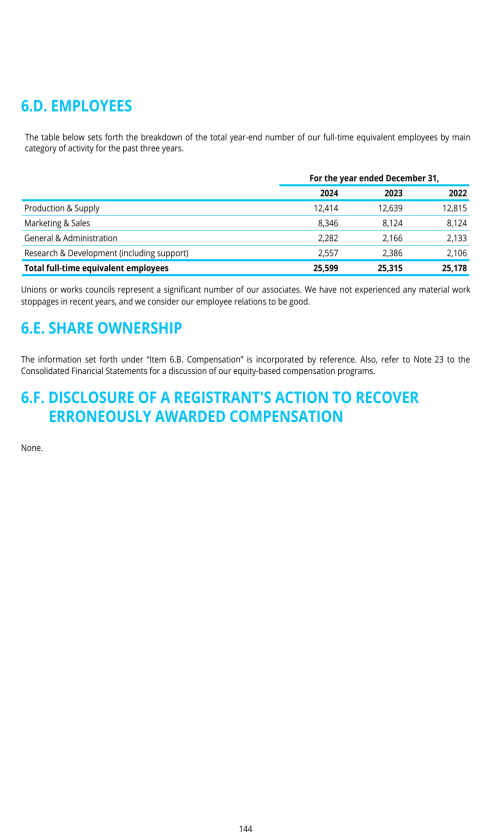
6.D. EMPLOYEES
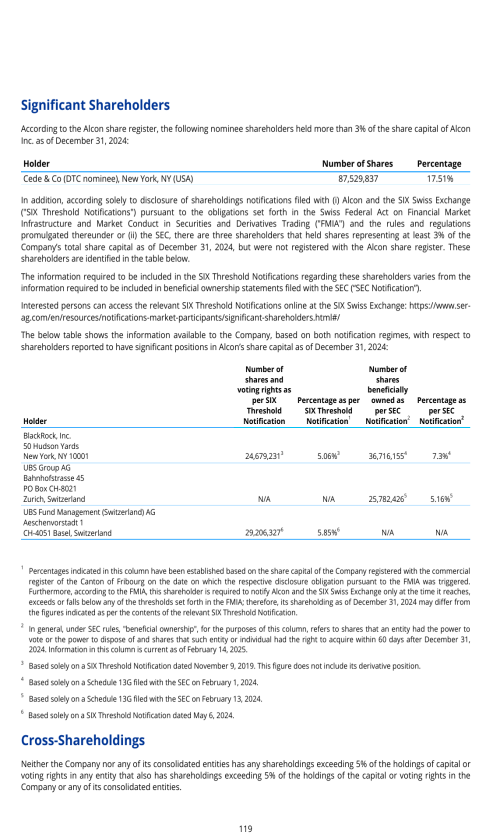
ITEM 7. MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS
7.A. MAJOR SHAREHOLDERS
7.B. RELATED PARTY TRANSACTIONS
7.C. INTERESTS OF EXPERTS AND COUNSEL
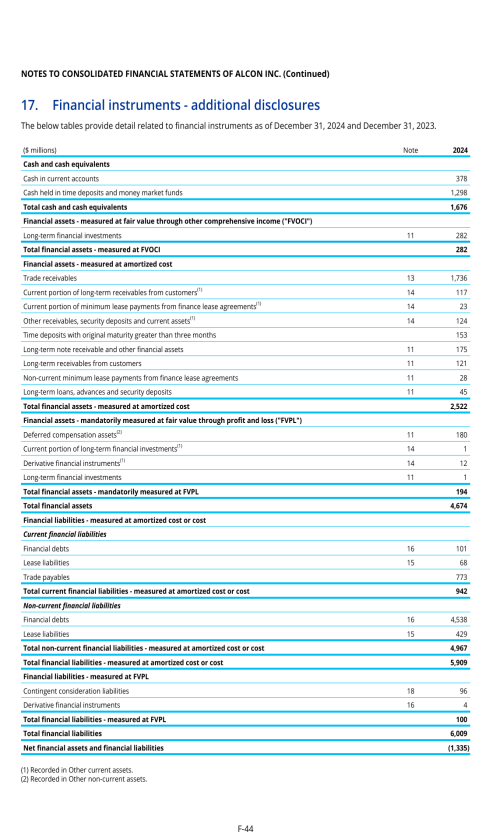
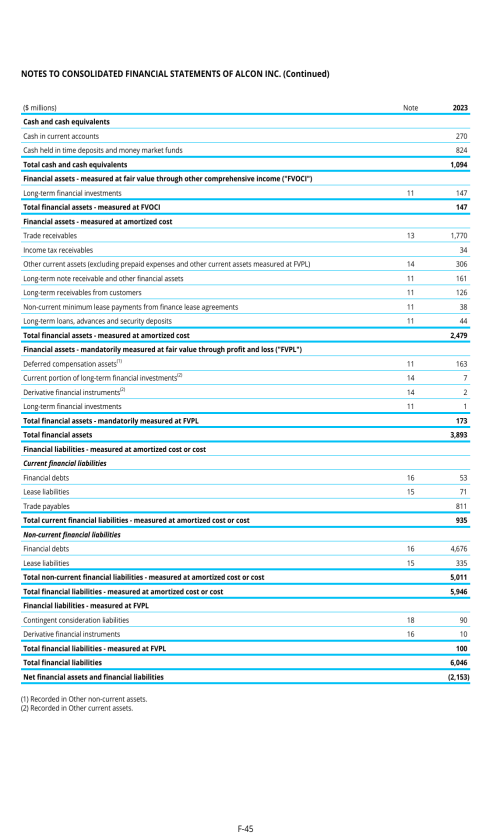
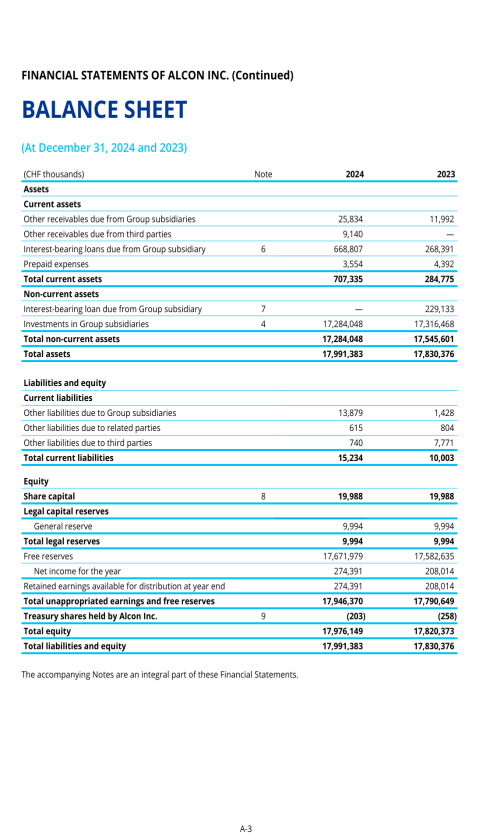
ITEM 8. FINANCIAL INFORMATION
8.A. CONSOLIDATED STATEMENTS AND OTHER FINANCIAL INFORMATION
Legal proceedings
Dividend policy
8.B. SIGNIFICANT CHANGES
ITEM 9. THE OFFER AND LISTING
9.A. OFFER AND LISTING DETAILS
9.B. PLAN OF DISTRIBUTION
9.C. MARKETS
9.D. SELLING SHAREHOLDERS
9.E. DILUTION
9.F. EXPENSES OF THE ISSUE
ITEM 10. ADDITIONAL INFORMATION
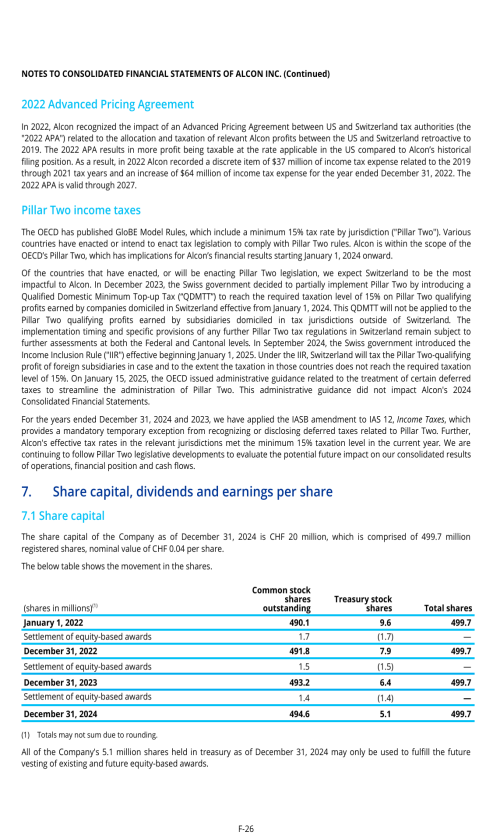
10.A. SHARE CAPITAL
10.B. MEMORANDUM AND ARTICLES OF ASSOCIATION
10.C. MATERIAL CONTRACTS
10.D. EXCHANGE CONTROLS
10.E. TAXATION
10.F. DIVIDENDS AND PAYING AGENTS
10.G. STATEMENTS BY EXPERTS
10.H. DOCUMENTS ON DISPLAY
10.I. SUBSIDIARY INFORMATION
ITEM 11. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
ITEM 12. DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES
12.A. DEBT SECURITIES
12.B. WARRANTS AND RIGHTS
12.C. OTHER SECURITIES
12.D. AMERICAN DEPOSITARY SHARES
PART II
ITEM 13. DEFAULTS, DIVIDEND ARREARAGES AND DELINQUENCIES
ITEM 14. MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS
ITEM 15. CONTROLS AND PROCEDURES
Management's Annual Report on Internal Control Over Financial Reporting
Changes in Internal Control Over Financial Reporting
ITEM 16A. AUDIT COMMITTEE AND FINANCIAL EXPERT
ITEM 16B. CODE OF ETHICS
ITEM 16C. PRINCIPAL ACCOUNTANT FEES AND SERVICES
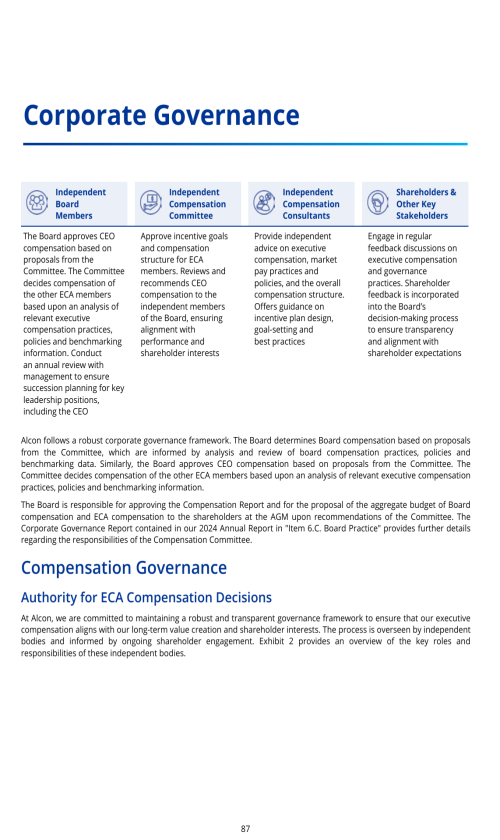
ITEM 16D. EXEMPTIONS FROM THE LISTING STANDARDS FOR AUDIT COMMITTEES
ITEM 16E. PURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS
ITEM 16F. CHANGE IN REGISTRANT'S CERTIFYING ACCOUNTANT
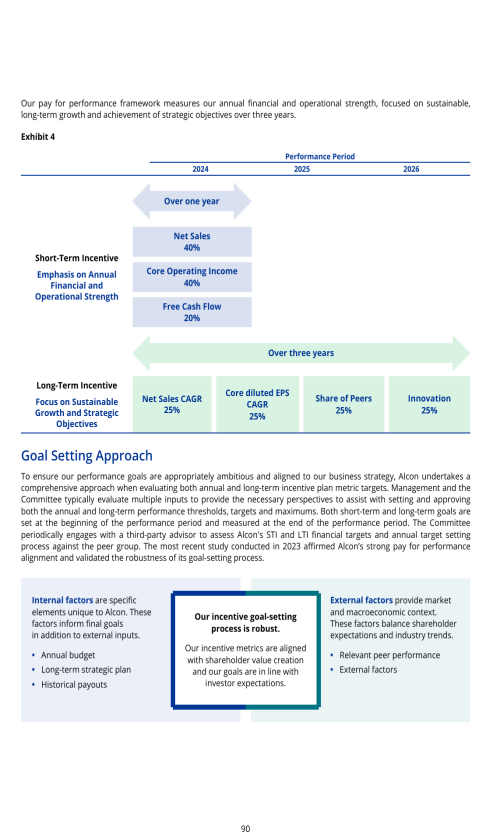
ITEM 16G. CORPORATE GOVERNANCE
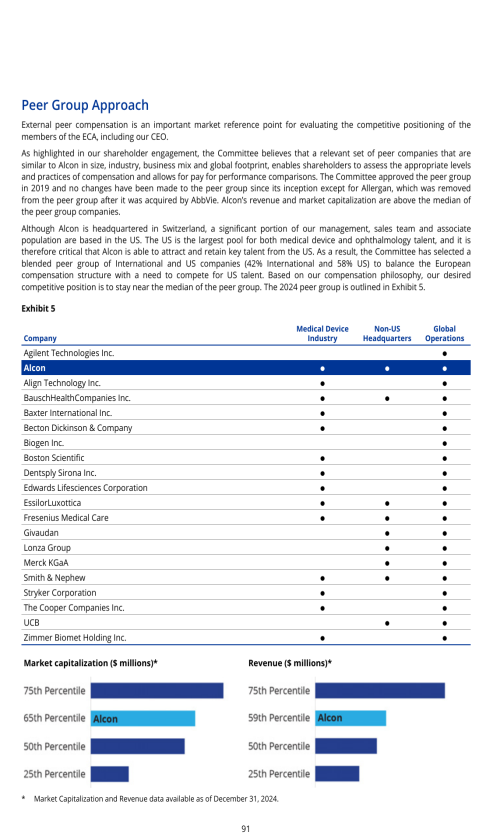
ITEM 16H. MINE SAFETY DISCLOSURE
ITEM 16I. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
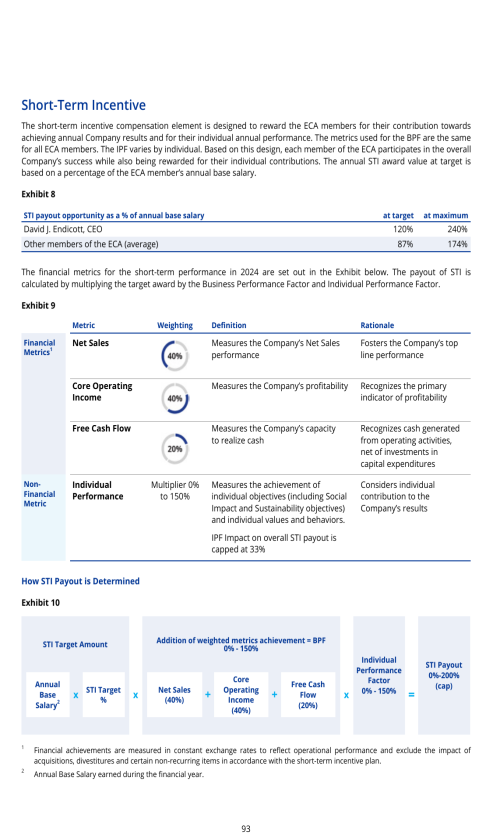
ITEM 16J. INSIDER TRADING POLICIES
ITEM 16K. CYBERSECURITY
PART III
Swiss Annual Report - SEC exhibits
CONSOLIDATED FINANCIAL STATEMENTS
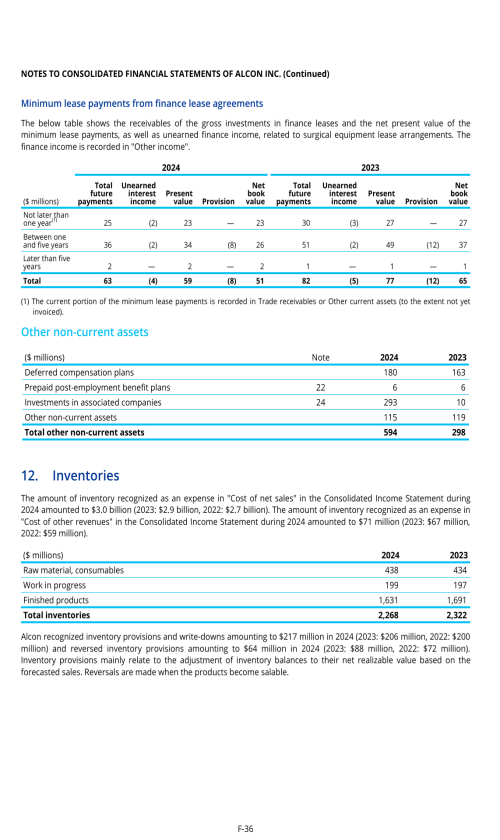
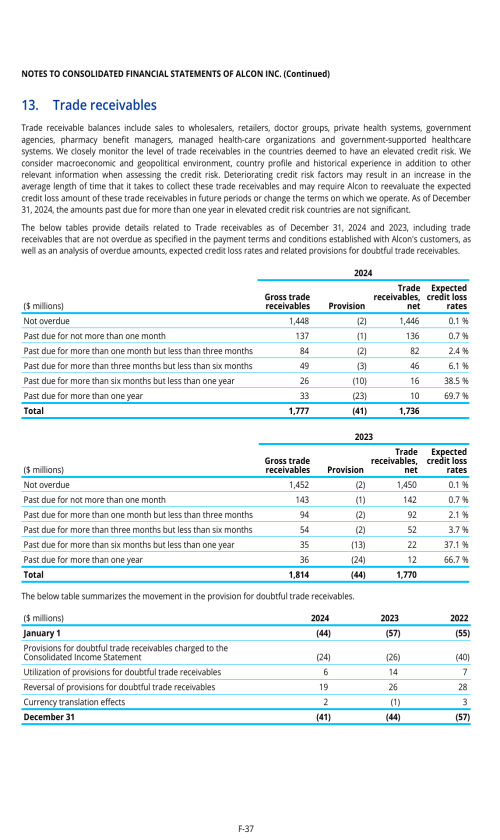
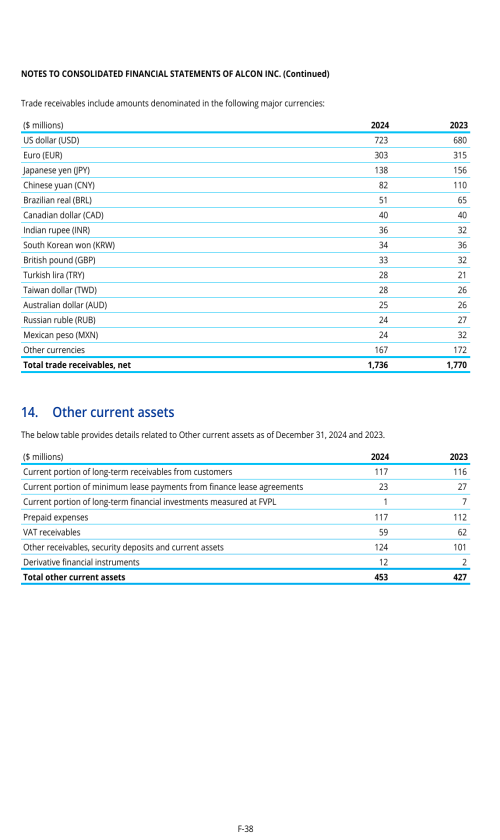
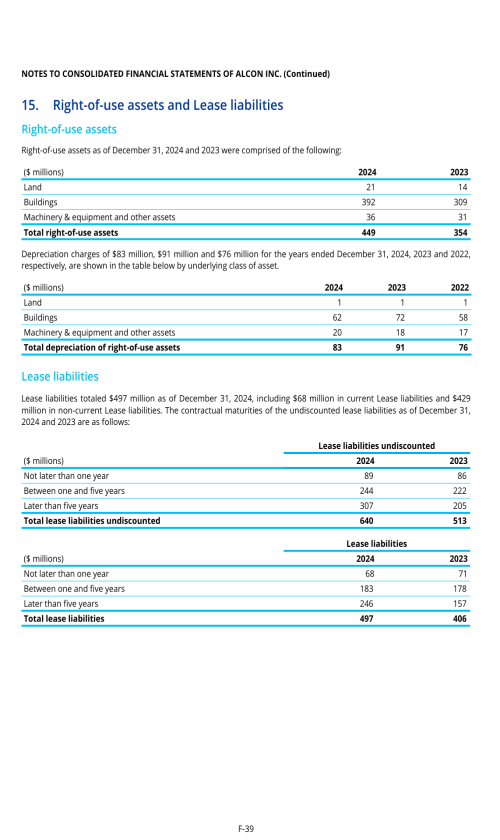
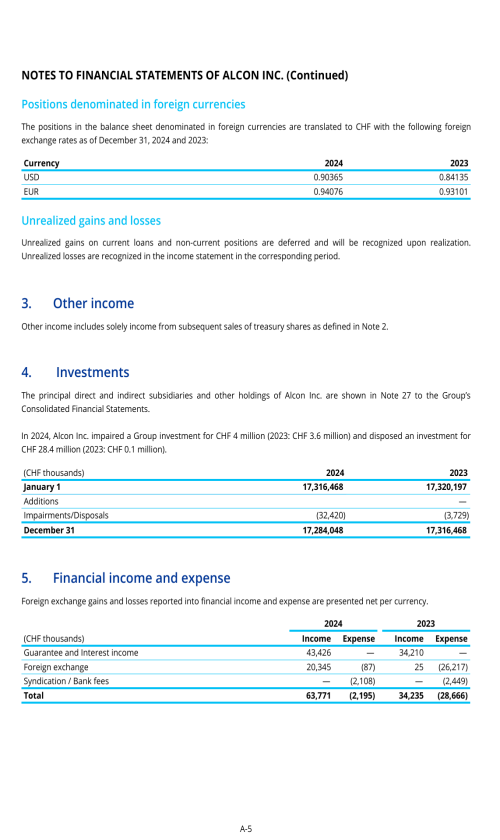
Footnotes
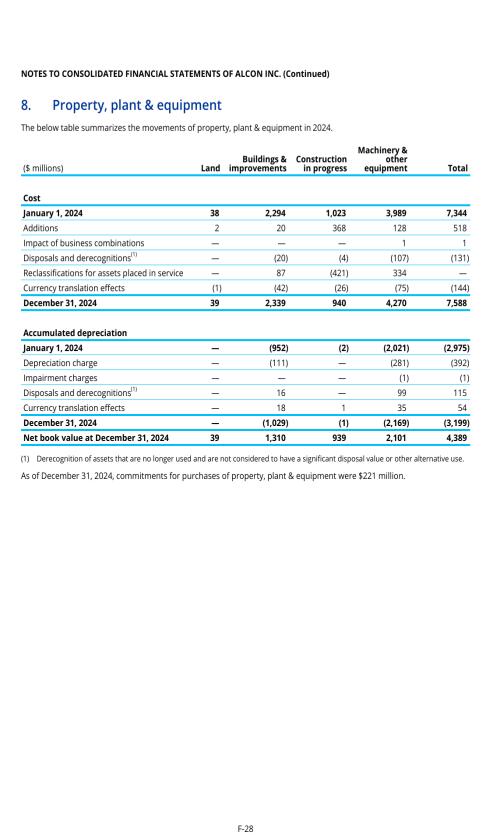
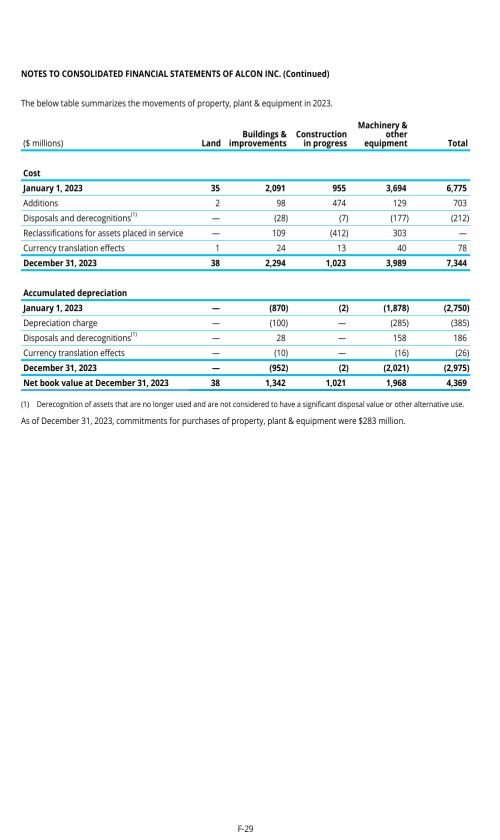
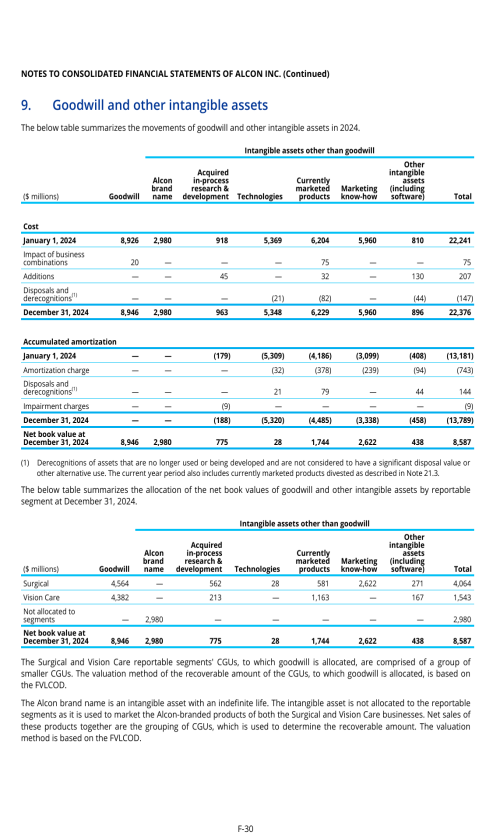
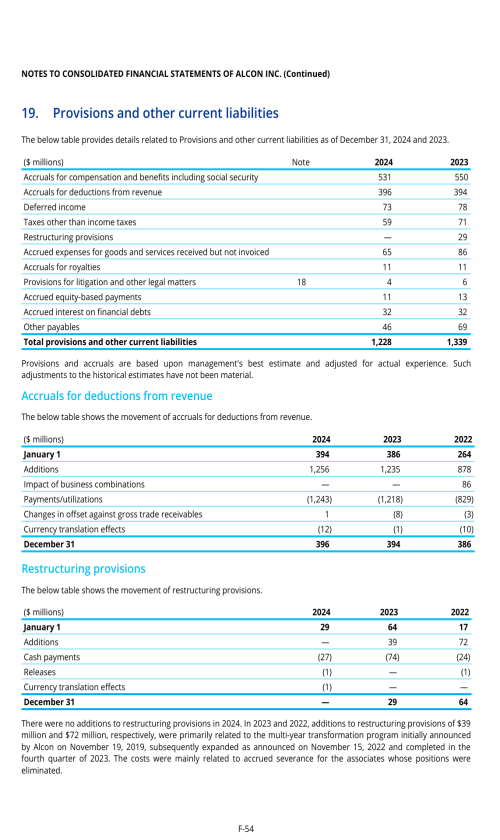
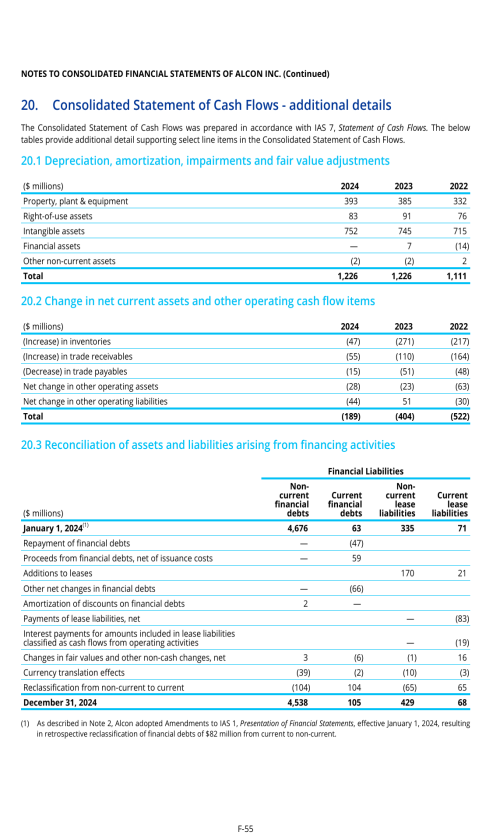
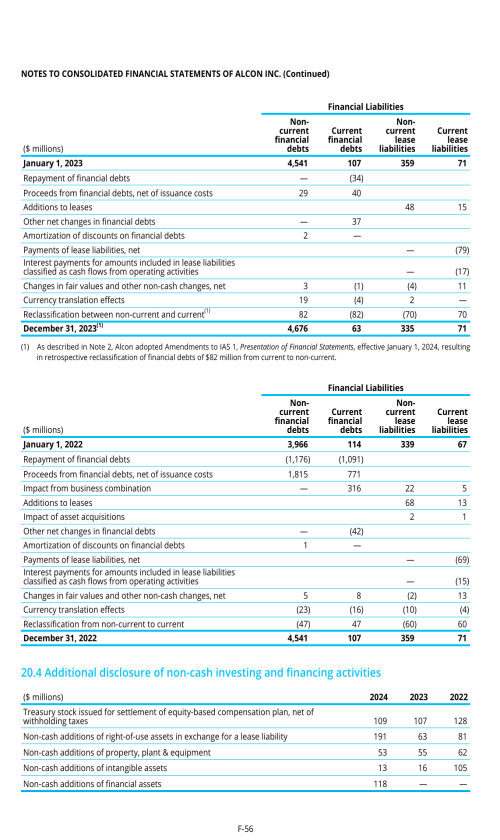
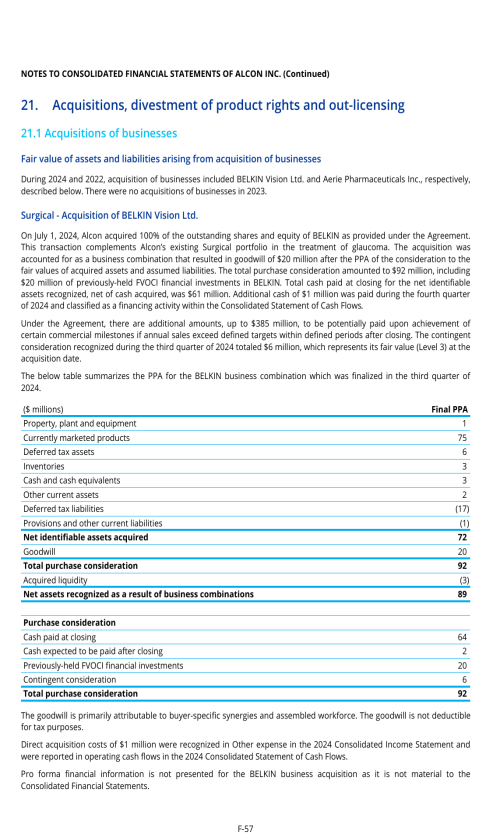
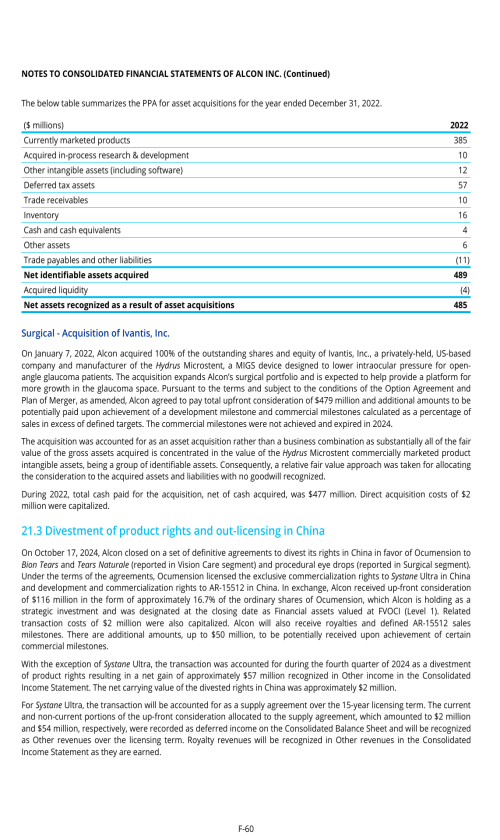
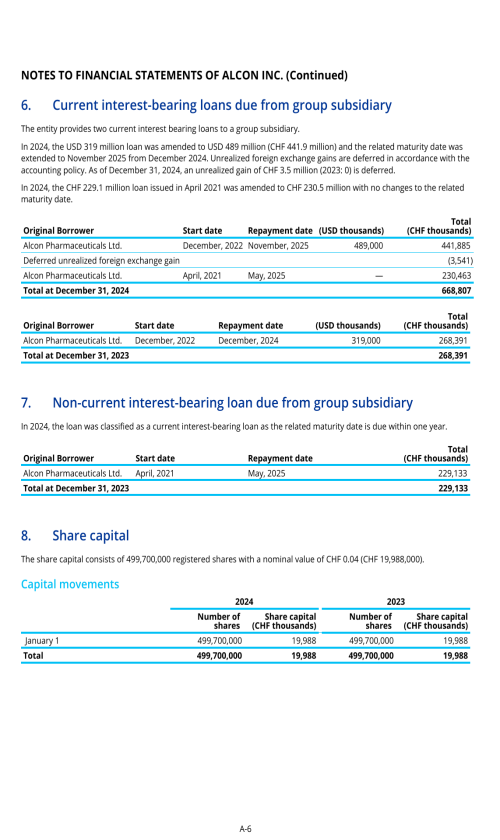
1. Description of business
2. Selected accounting policies
3. Significant transactions
4. Segment information
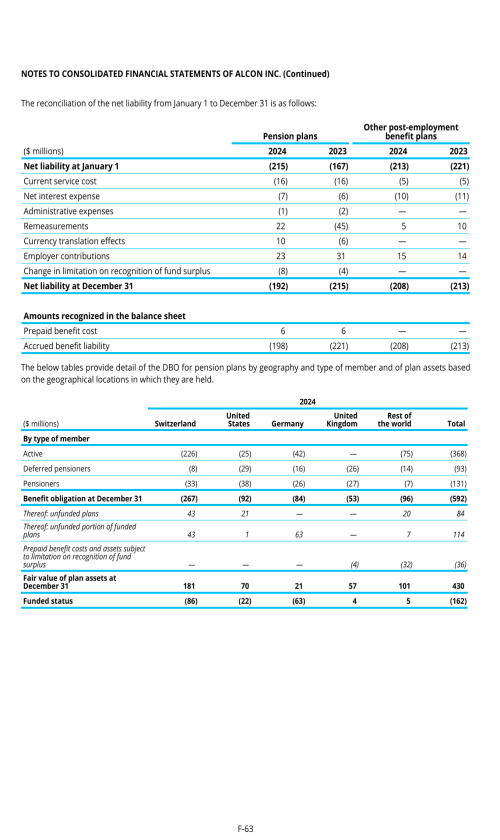
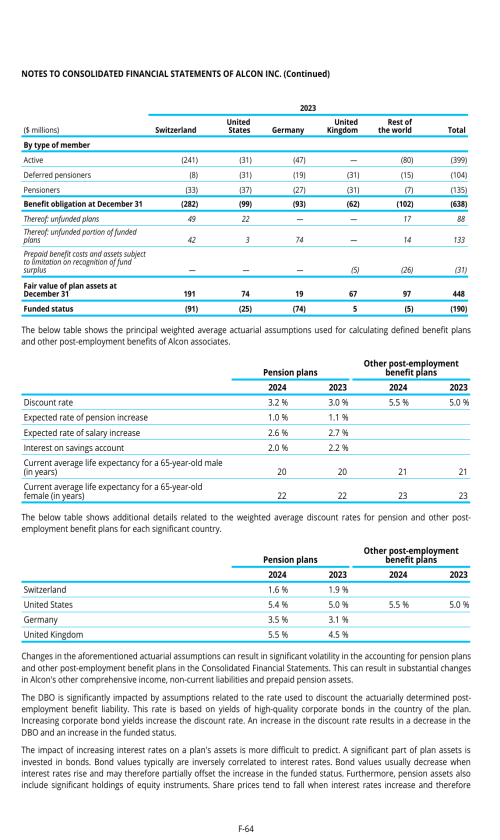
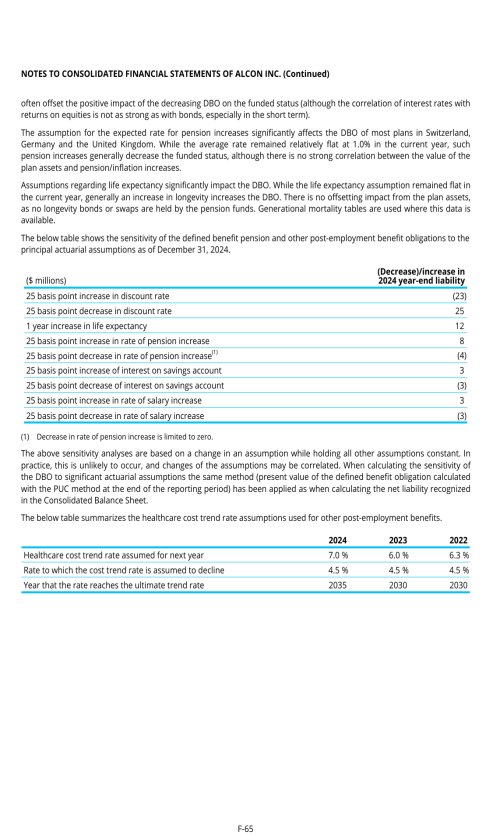
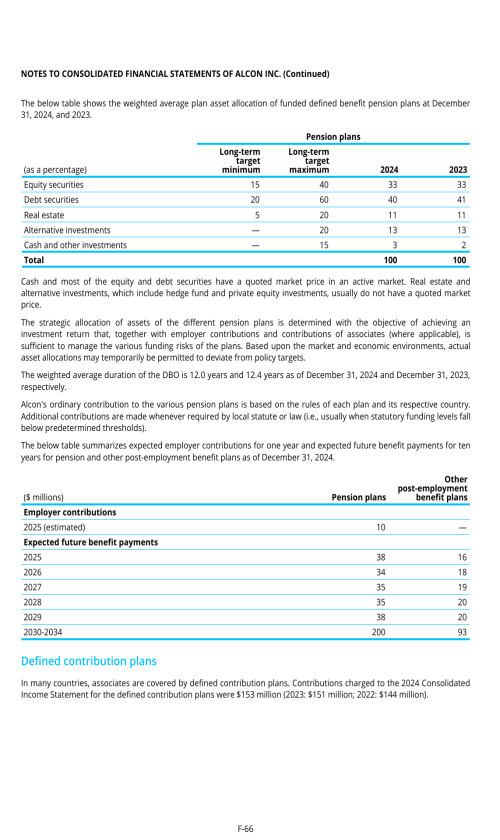
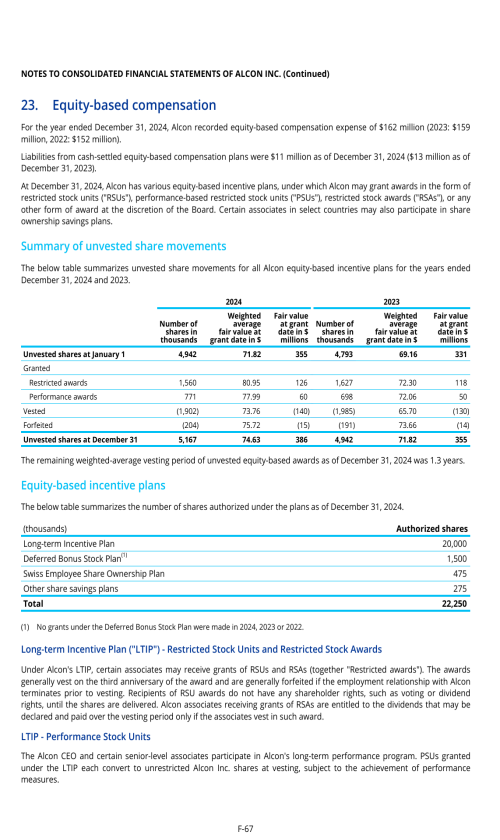
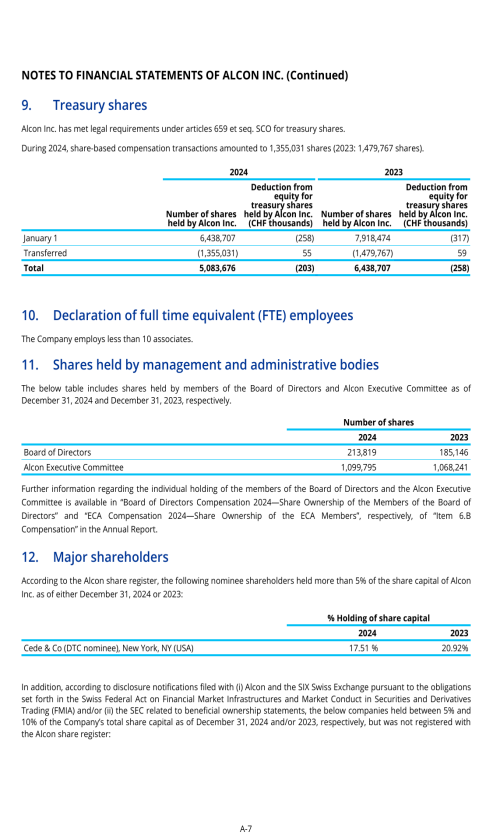
22. Post-employment benefits for associates
26. Subsequent events
Swiss Annual Report - FINANCIAL STATEMENTS
1. Introduction
2. Accounting policies
Pages
Search
Index
Swiss Annual Report_Compiled for BoD Bring-down_2.24.2024
ALC 20F 2024 at Q4 (SWISS ANNUAL REPORT - BoD BRING-DOWN)
INTRODUCTION AND USE OF CERTAIN TERMS
MARKET INFORMATION
SPECIAL NOTE ABOUT FORWARD-LOOKING STATEMENTS
PART I
ITEM 1. IDENTITY OF DIRECTORS, SENIOR MANAGEMENT AND ADVISERS
1.A. DIRECTORS AND SENIOR MANAGEMENT
1.B. ADVISERS
1.C. AUDITORS
ITEM 2. OFFER STATISTICS AND EXPECTED TIMETABLE
ITEM 3. KEY INFORMATION
3.A. RESERVED
3.B. CAPITALIZATION AND INDEBTEDNESS
3.C. REASONS FOR THE OFFER AND USE OF PROCEEDS
3.D. RISK FACTORS
ITEM 4. INFORMATION ON THE COMPANY
4.A. HISTORY AND DEVELOPMENT OF THE COMPANY
4.B. BUSINESS OVERVIEW
ITEM 5. OPERATING AND FINANCIAL REVIEW AND PROSPECTS
5.A. OPERATING RESULTS
Overview
Basis of preparation
Items you should consider
Segment description
Opportunity and risk summary
Components of results of operations
Critical accounting policies and estimates
Factors affecting comparability of period to period results of operations
Effects of currency fluctuations
SUPPLEMENTARY INFORMATION - DEFINITIONS AND RECONCILIATIONS OF NON-IFRS MEASURES
Non-IFRS measures as defined by the Company
ITEM 6. DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES
6.A. DIRECTORS AND SENIOR MANAGEMENT
6.C. BOARD PRACTICES
Information and Control System of the Board vis-à-vis the Management
Information to the Board of Directors
Alcon Management Information System
Internal Audit
Internal Control System
Risk Management
Compliance Function
Changes of Control and Defense Measures
Auditors
6.D. EMPLOYEES
ITEM 7. MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS
7.A. MAJOR SHAREHOLDERS
7.B. RELATED PARTY TRANSACTIONS
7.C. INTERESTS OF EXPERTS AND COUNSEL
ITEM 8. FINANCIAL INFORMATION
8.A. CONSOLIDATED STATEMENTS AND OTHER FINANCIAL INFORMATION
Legal proceedings
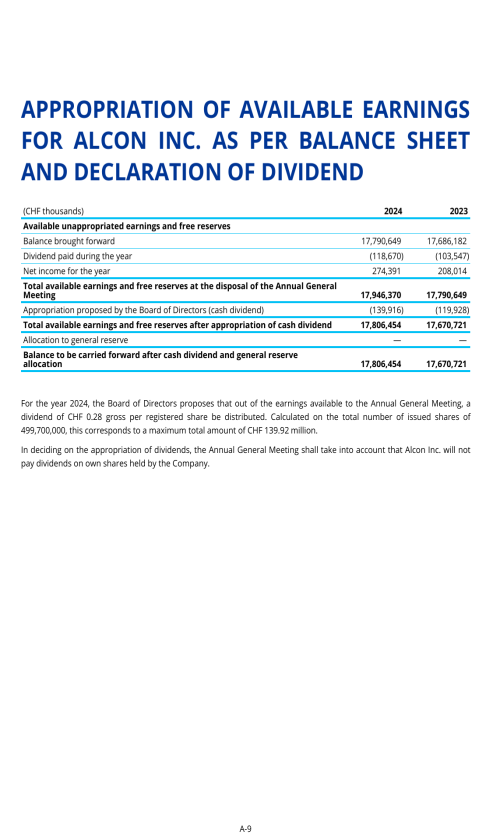
Dividend policy
8.B. SIGNIFICANT CHANGES
ITEM 9. THE OFFER AND LISTING
9.A. OFFER AND LISTING DETAILS
9.B. PLAN OF DISTRIBUTION
9.C. MARKETS
9.D. SELLING SHAREHOLDERS
9.E. DILUTION
9.F. EXPENSES OF THE ISSUE
ITEM 10. ADDITIONAL INFORMATION
10.A. SHARE CAPITAL
10.B. MEMORANDUM AND ARTICLES OF ASSOCIATION
10.C. MATERIAL CONTRACTS
10.D. EXCHANGE CONTROLS
10.E. TAXATION
10.F. DIVIDENDS AND PAYING AGENTS
10.G. STATEMENTS BY EXPERTS
10.H. DOCUMENTS ON DISPLAY
10.I. SUBSIDIARY INFORMATION
ITEM 11. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
ITEM 12. DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES
12.A. DEBT SECURITIES
12.B. WARRANTS AND RIGHTS
12.C. OTHER SECURITIES
12.D. AMERICAN DEPOSITARY SHARES
PART II
ITEM 13. DEFAULTS, DIVIDEND ARREARAGES AND DELINQUENCIES
ITEM 14. MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS
ITEM 15. CONTROLS AND PROCEDURES
Management's Annual Report on Internal Control Over Financial Reporting
Changes in Internal Control Over Financial Reporting
ITEM 16A. AUDIT COMMITTEE AND FINANCIAL EXPERT
ITEM 16B. CODE OF ETHICS
ITEM 16C. PRINCIPAL ACCOUNTANT FEES AND SERVICES
ITEM 16D. EXEMPTIONS FROM THE LISTING STANDARDS FOR AUDIT COMMITTEES
ITEM 16E. PURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS
ITEM 16F. CHANGE IN REGISTRANT'S CERTIFYING ACCOUNTANT
ITEM 16G. CORPORATE GOVERNANCE
ITEM 16H. MINE SAFETY DISCLOSURE
ITEM 16I. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
ITEM 16J. INSIDER TRADING POLICIES
ITEM 16K. CYBERSECURITY
PART III
Swiss Annual Report - SEC exhibits
CONSOLIDATED FINANCIAL STATEMENTS
Footnotes
1. Description of business
2. Selected accounting policies
3. Significant transactions
4. Segment information
22. Post-employment benefits for associates
26. Subsequent events
Swiss Annual Report - FINANCIAL STATEMENTS
1. Introduction
2. Accounting policies
Pages
Search
55 / 274

DAILIES AquaComfort PLUS, our most affordable daily disposable contact lens in monofocal, astigmatism-correcting and
multifocal options, delivers dependable performance by working with tears to release moisture with every blink. This lens
is designed for value-conscious wearers who want the flexibility and simplicity of a daily disposable lens.
Air Optix, our more affordable monthly replacement product line, features SiHy contact lenses in monofocal, astigmatism-
correcting and multifocal options, as well as Air Optix Colors and Air Optix plus HydraGlyde contact lenses. Air Optix plus
HydraGlyde brings together two innovative technologies—SmartShield technology and HydraGlyde moisture matrix—for a
unique combination of deposit protection and longer-lasting lens surface moisture.
We continue to experience market growth driven by trade-up to premium lenses, expansion of toric and multifocal
specialty lenses, as well as increasing penetration in emerging markets.
Our key brands in our ocular health portfolio include the Systane family of artificial tear and related dry eye products,
Pataday family of eye allergy products, as well as the Opti-Free and Clear Care lines of multi-purpose and hydrogen
peroxide disinfecting solutions, respectively.
Alcon currently holds a market leading position in artificial tears. We continue to focus on core product performance while
increasing promotion behind a best-in-class innovation portfolio under the brand leadership of Systane artificial tears. The
Systane portfolio is a comprehensive offering of ocular health solutions, most of which are indicated for the temporary
relief of burning and irritation due to dryness of the eye. The Systane portfolio includes products for daily and nighttime
relief, as well as products for discomfort associated with contact lens wear. In 2021, we continued significant international
expansion for Systane Ultra multi-dose preservative-free ("MDPF") and Systane Hydration MDPF. In 2022, we launched a
preservative-free formulation of Systane Complete. By adding the option of MDPF presentations to our portfolio, we
address a key need by many eye care practitioners for effective dry eye relief without preservatives. In 2025, we plan to
extend our Systane line with a new formulation that combines the strength of Systane Complete nano-lipids with hyaluronic
acid called Systane Pro to provide relief from more severe dry eye symptoms. Systane Pro will be the first Systane
lubricating drop with HA and lipids for all types of dry eye relief.
Previously available only by prescription, in 2020, we began to offer the Pataday family of allergy relief eye drops over-the-
counter in the US. Pataday Twice Daily Relief, Pataday Once Daily Relief and Pataday Once Daily Extra Strength eye drops
each contains olopatadine, the number one doctor-prescribed active ingredient for eye allergy relief.
In 2021, we began our expansion into the ophthalmic pharmaceutical space by acquiring the exclusive US
commercialization rights to Simbrinza, a fixed combination of a carbonic anhydrase inhibitor and an alpha-2 adrenergic
receptor agonist indicated for the reduction of elevated intraocular pressure ("IOP") in patients with open-angle glaucoma
or ocular hypertension. We then acquired Eysuvis, a corticosteroid indicated for the short-term (up to two weeks)
treatment of the signs and symptoms of dry eye disease, and Inveltys, a corticosteroid indicated for the treatment of post-
operative inflammation and pain following ocular surgery, from Kala Pharmaceuticals, Inc. in July 2022. In November 2022,
to complement our previous acquisitions, we acquired Aerie Pharmaceuticals, Inc. The Aerie transaction adds on-market
products Rhopressa, a once-daily eye drop that contains netarsudil, a Rho kinase (ROCK) inhibitor specifically designed to
target a diseased trabecular meshwork believed to be the main cause of elevated IOP in open-angle glaucoma and ocular
hypertension, and Rocklatan, a once-daily eye drop that is a fixed-dose combination of latanoprost, a prostaglandin analog
(PGA), and netarsudil, as well as a pipeline of products, including AR-15512, a Phase 3 product candidate for dry eye
disease, and R&D capabilities to expand our ophthalmic pharmaceutical presence. We are planning to launch AR-15512 in
2025, pending FDA approvals and other required registrations.
Alcon is also a market leader in contact lens care in both multi-purpose (Opti-Free PureMoist) and hydrogen peroxide
solutions (Clear Care and AOSEPT PLUS). The vast majority of our contact lens care products are comprised of disinfecting
solutions to remove harmful micro-organisms on contact lenses, with a smaller amount of sales coming from contact lens
rewetting drops to improve wearing comfort for contact lenses. We benefit from strong synergies between our contact
lens business and our contact lens care products.
Finally, our ocular health portfolio also includes artificial tear and related dry eye products marketed under the Tears
Naturale and Genteal brands, products for the temporary relief of ocular itching due to ocular allergies marketed under the
43